Drug quality analysis platform
The platform has complete software and hardware resources, experienced research talents and advanced solid-state research and analysis instruments, such as HPLC, GC, LC-mass, Pre-HPLC and others, which can carry out quality research on APIs in the following aspects:
According to the pharmacopoeia standards of various countries or the analysis methods independently developed, all kinds of items are tested, mainly including the following categories:
|
Physical constant |
Melting point, density, pH value, optical rotation, conductivity, TOC, etc. |
|
Identification |
UV, IR, HPLC, etc. |
|
Limit |
Moisture, weight loss on drying, residue ignition, total ash, elemental impurities, residual solvents and related substances |
|
Characteristic check |
Appearance color, solution color, solution clarity, visible foreign matter, etc. |
|
Content |
Content determination (including chromatography, volumetric method and gravimetric method) |
|
Solid state analysis |
Crystal form, particle size, packing density, bulk density and rheological fluidity |
The main analytical instruments are as follows:
|
Chromatographic class |
HPLC (VWD, DAD), flame ionizationdetector (FID) |
|
Spectral class |
IR, UV |
|
Mass spectrometry |
HPLC-mass, GC-mass, ICP-mass |
|
Titration class |
Potentiometric titrator, Karl Fischer water titrator |
|
Physics and Chemistry |
Melting point meter, pH meter, balance |
|
Solid state class |
PSD, XRD, DSC, TGA |
Based on the requirements of ICH guidelines and pharmacopoeias of various countries, the analysis methods of elemental impurities in APIs were developed, confirmed and verified to provide the basis for risk assessment. The main instrument used was ICP-mass.
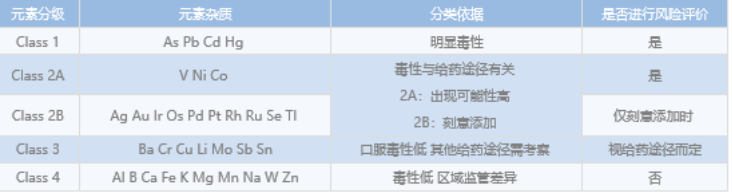
In recent years, regulatory agencies in various countries, such as ICH, FDA, EMA, etc., have more clear requirements for genotoxic impurities. More and more pharmaceutical companies focus on the control and detection of genotoxic impurities in the process of new drug research and development. According to ICH M7 "Assessment and Control of DNA Reactive (Mutagenic) Immunities in Pharmaceuticals to Limit Potential Carcinogenic Risk", genotoxic impurities are studied. According to the different limits of genotoxic impurities, UPLC/UV and UPLC/QDa (single quadrupole rod) instruments can be used respectively.
Stability research is based on systematic research and understanding of APIs or preparations and their production processes, Through design experiments, the quality characteristics of raw materials or preparations change with time under the influence of various environmental factors (such as temperature, humidity, light irradiation, etc.), and provide supporting information for the determination of prescription, process, packaging, storage conditions and re-inspection period/validity period of drugs; Including influencing factor test, accelerated test and long-term sample retention test. The platform has MELT stability test chamber and other equipment.


